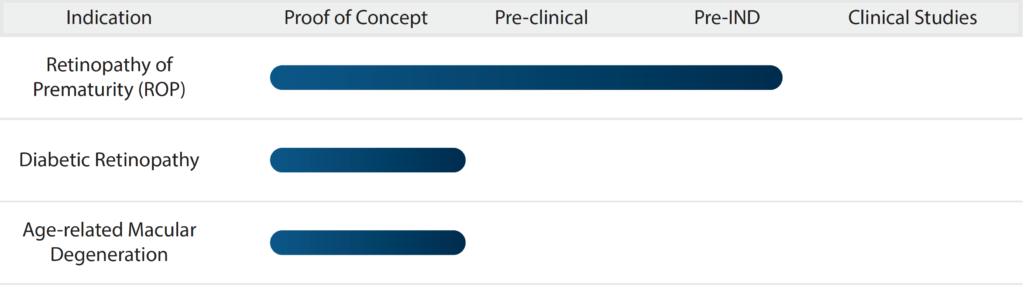
Pipeline
PR1P Development Pipeline
The Orpheus Solution
To redress the risk of blindness in premature neonates, Orpheus proposes the use of a novel peptide therapeutic, PR1P, which can be administered prophylactically to prevent ROP development long before the damaging pathologies of ROP take place.
Retinopathy of prematurity (ROP)
The development of the retinal layers and its vasculature begins around 16 weeks of gestation; however, completion of this vascularization process only occurs at full term, or approximately 40 weeks of gestation. Therefore, for babies born prematurely, the retinal vasculature is both incompletely developed and highly susceptible to pathological maldevelopment. Removal from the physiological environment in utero introduces a multitude of changes, further confounding the otherwise normal developmental progression.
Among the most notable of these environmental changes is blood oxygen levels. Specifically, premature neonates may not acquire adequate oxygen through breathing alone, necessitating oxygen supplementation and sometimes more invasive respiratory support. While these interventions have drastically improved survival rates for premature babies, they also introduce high levels of oxygen that inhibit normal retinal vascular development. This means elevated retinal oxygen will mimic the effects of over-abundant blood vessels, diminishing the expression of the central vascular development regulator, vascular endothelial growth factor (VEGF). As VEGF levels in the retina decline, the retinal vascular development stalls, leaving large avascular zones devoid of blood vessels.
Later, as these neonates receive less invasive respiratory support, oxygen levels in the retina precipitously decline in these avascular zones, leading to a resurgence in VEGF expression. It is at this stage that ROP can progress to blindness as new and highly disorganized blood vessel networks are formed under the influence of VEGF. In these babies, the irregular vascular networks proliferate uncontrollably, a process called neovascularization, which can result in tractional retinal detachment.
As ROP became increasingly well-characterized after the introduction of oxygen supplementation in the 1930s and 1940s, screening for ROP became commonplace and is performed routinely today in premature neonates. When identified at earlier stages, ROP progression can be halted with laser, cryosurgery, or anti-VEGF therapies. However, it is also important to note that these therapies are administered after retinal damage has already occurred and carry the risk of additional ocular damage and poor outcomes.